CONTROLLED PRESCRIPTION PROGRAM
The Controlled Prescription Program (CPP) aims to reduce inappropriate prescribing of selected controlled drugs and to prevent forgeries. Prescriptions for the controlled drugs specified in the program must be written on the duplicate prescription pad specially developed for this purpose.
Quick Links |
Program Partners
- BC College of Nurses and Midwives
- Britsh Columbia College of Oral Health Professionals
- College of Pharmacists of BC
- College of Physicians & Surgeons of BC
- College of Veterinarians of BC
- Ministry of Health (PharmaCare Program)
Representatives from each of these organizations make up the Controlled Prescription Program Advisory Committee which is responsible for regularly reviewing and updating the Controlled Prescription Program requirements and drug list, and making recommendations regarding drugs that should require a duplicate prescription, and the information that should be provided by registrants of each college on a duplicate prescription.
In addition, the committee provides a collaborative forum to discuss common concerns and knowledge related to prescribing of drugs with a high-risk profile. Best practices discussed by the forum also help guide development of related College standards for health professionals.
Under section 19(6)(a) of the College of Pharmacists of BC’s Bylaws under the Pharmacy Operations and Drug Scheduling Act, the controlled prescription form must be approved by the Boards of both the College of Pharmacists of BC and the College of Physicians and Surgeons of British Columbia.
In addition, under section 22(1) of the Pharmacy Operations and Drug Scheduling Act the College of Pharmacists of BC is also responsible for making amendments as needed to the Drug Schedules Regulation, which specifies the terms and conditions for the sale of drugs in the province.
How the Program Works
The selected drugs included in the program may only be prescribed in writing using a special Controlled Prescription Program duplicate pad printed for the purpose.
Once the prescription is written, the prescriber retains the bottom copy marked “PRESCRIBERS COPY” and provides the patient with the original identified as “PHARMACY COPY,” which the patient gives to the pharmacist.
Note: Controlled Prescription Program duplicate prescription pads must still be used when using Electronic Medical Records (EMRs). As with all prescriptions, prescribers must ensure that all fields on Controlled Prescription Program forms are completed correctly including one generated from an EMR.
The Controlled Prescription Program also includes requirements and guidance on:
- ordering duplicate prescription pads
- safe keeping and reporting lost, stolen, or forged prescription pads
- voiding a duplicate prescription pad
- record retention
- prescription from delivery options, including mailing and receipt of forms to pharmacy
Drugs Included in the Program
The list of drugs covered by the program has been agreed to by all the program participants. Unless otherwise specified, both single-entity products and preparations or mixtures of the scheduled drugs require the use of Controlled Prescription Program forms.
Drugs included in the program are listed as Schedule 1A drugs in the Drug Schedules Regulation under the Pharmacy Operations and Drug Scheduling Act.
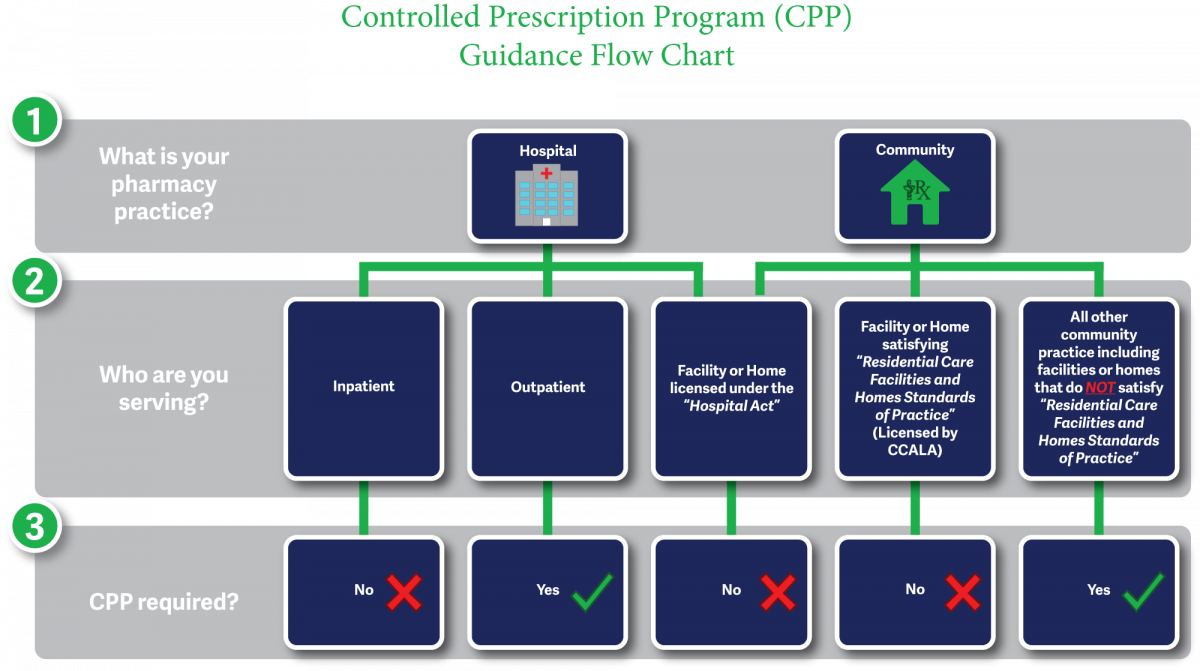
Prescriptions for long-term and extended-care facility patients do not require the use of Controlled Prescription Program forms.
Note: More than one strength of medication can be included on one Controlled Prescription Program form, provided the orders are legible.
| See the full list of Schedule 1A drugs within the Drug Schedules Regulation that require the use of a Controlled Prescription Program Form |
The following summary highlights many of the Schedule 1a drugs that drugs require the use of a Controlled Prescription Program form:
|
Alfentanil
|
Fentanyl
|
Morphine
|
| Anileridine |
Hydrocodone (Dihydrocodeinone)
|
Normethadone |
|
Buprenorphine
|
Hydromorphone (Dihydromorphinone)
|
Oxycodone
|
|
Butalbital
|
Levorphanol |
Pentazocine
|
|
Butorphanol
|
Meperidine (Pethidine)
|
Propoxyphene (Dextropropoxyphene)
|
|
Codeine when prescribed as a single entity, when included in a preparation containing 60 mg or more per dosage unit , or when included in a liquid preparation containing 1.6 mg or more per mL
|
Methadone
|
Sufentanil
|
|
Diacetylmorphine (heroin) |
Methaqualone |
Tapentadol
|
|
Ethchlorvynol
|
Note: product names are examples only and are not intended to represent a complete list of all products available.
The following drug products are not Controlled Prescription Program drugs and do NOT require the use of a Controlled Prescription Program form:
Controlled Prescription Program Form
The Controlled Prescription Program Form is a specialized duplicate prescription with additional security measures to prevent forgeries. The duplicate prescription forms are prepared by the Ministry of Health and are only available to the following practitioners through a secure ordering process:
- Dentists
- Nurse Practitioners
- Physicians
- Registered Midwives
- Registered Nurses
- Registered Psychiatric Nurses
- Veterinarians
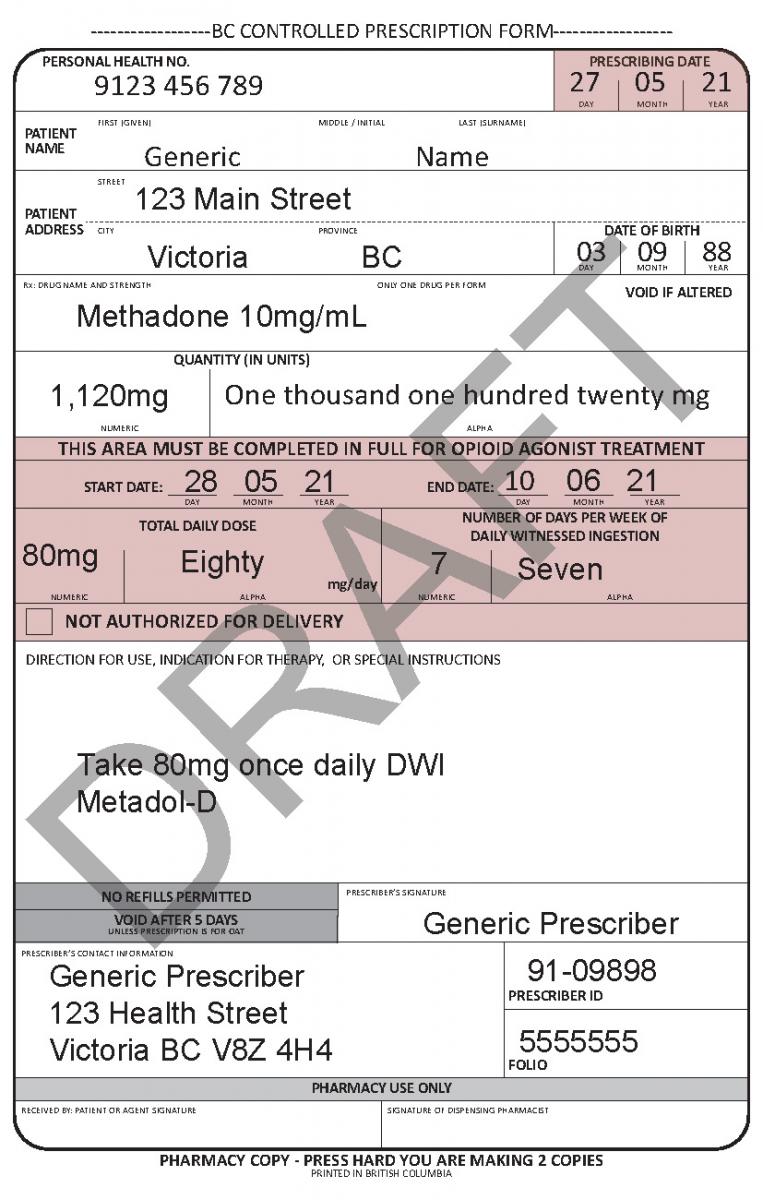
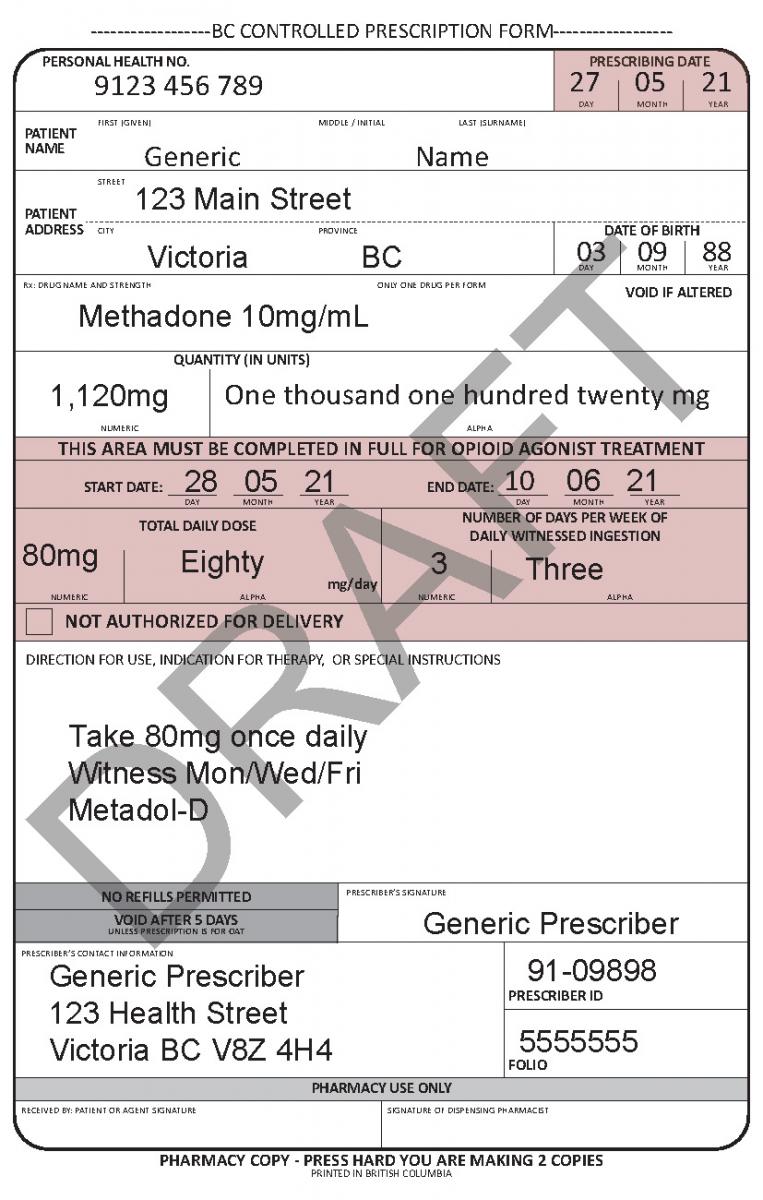
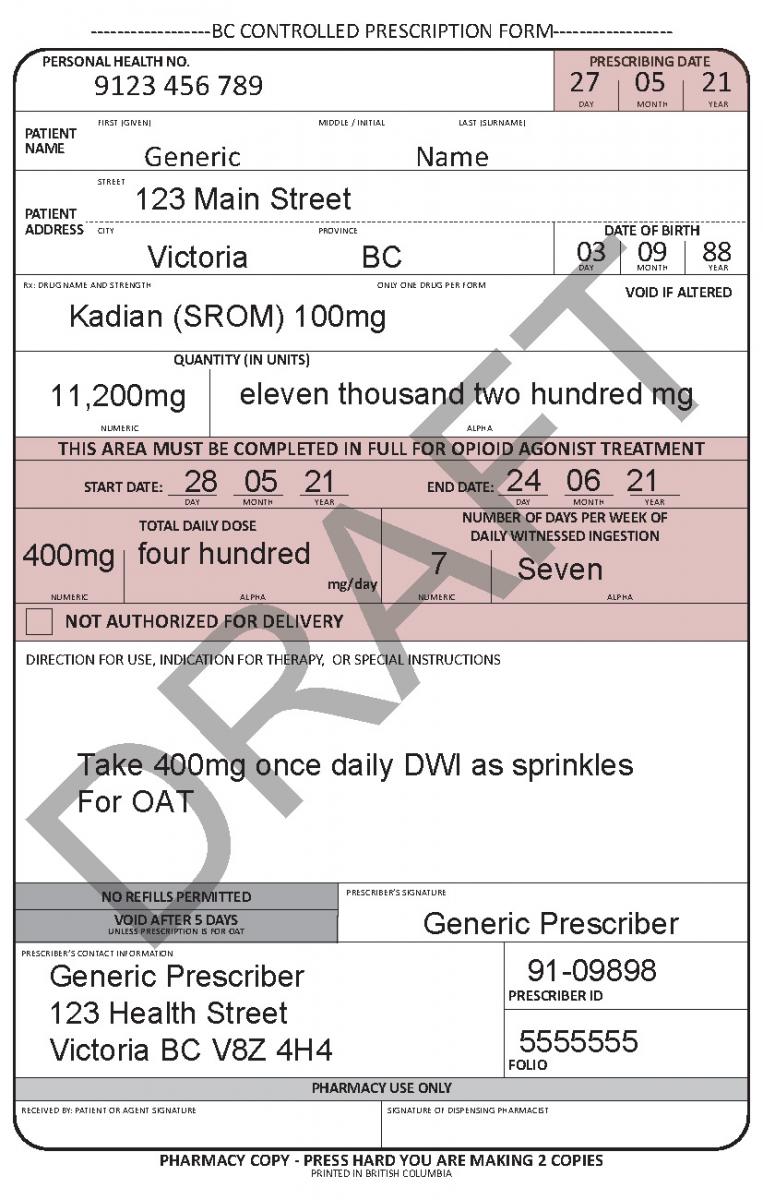
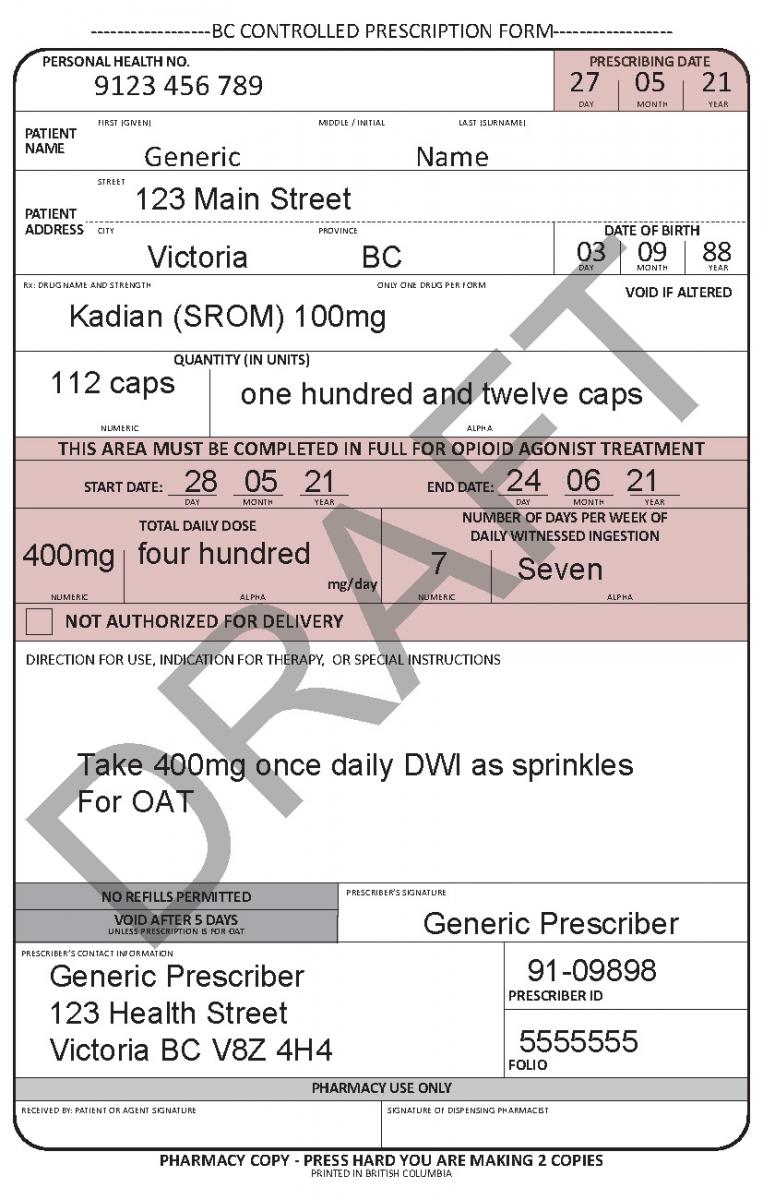
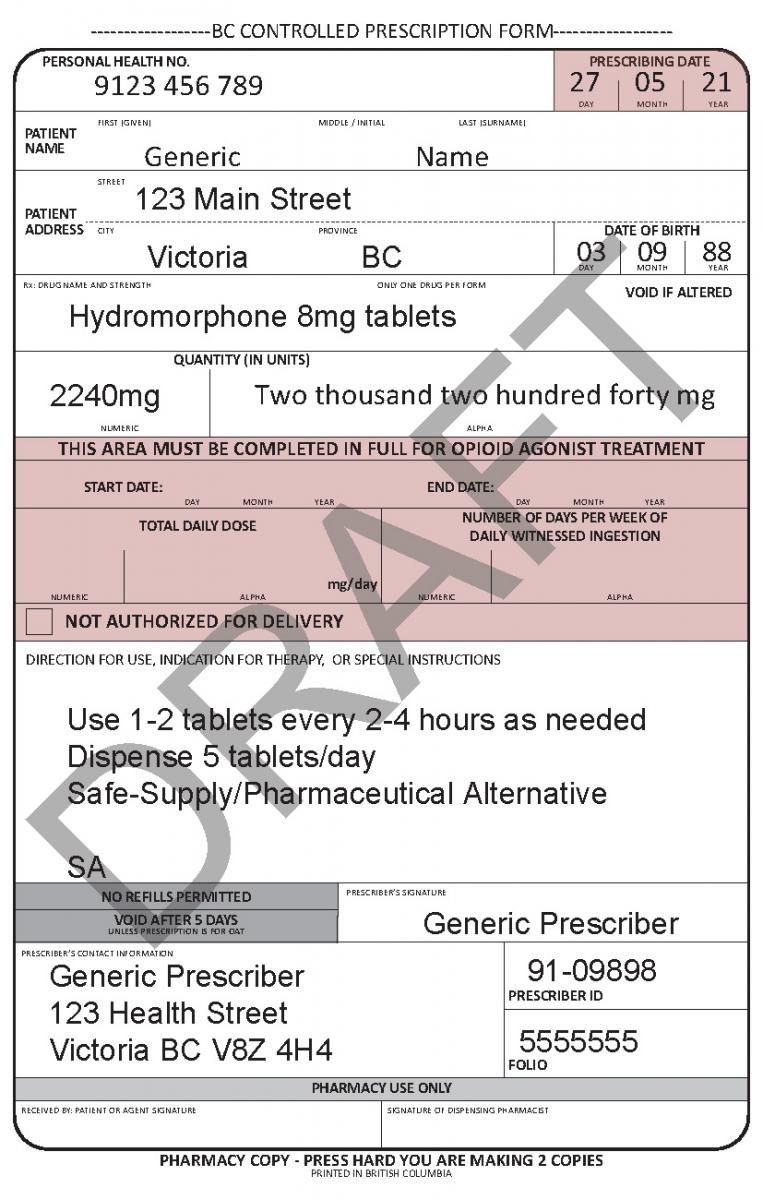
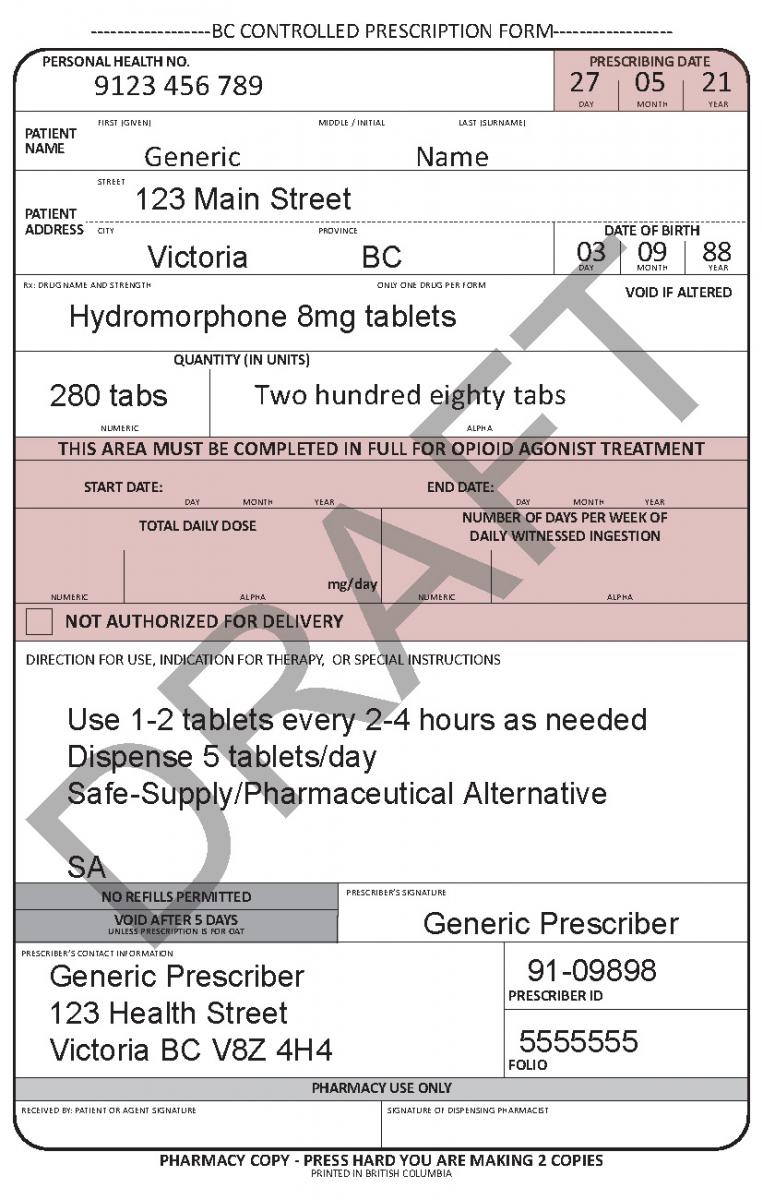
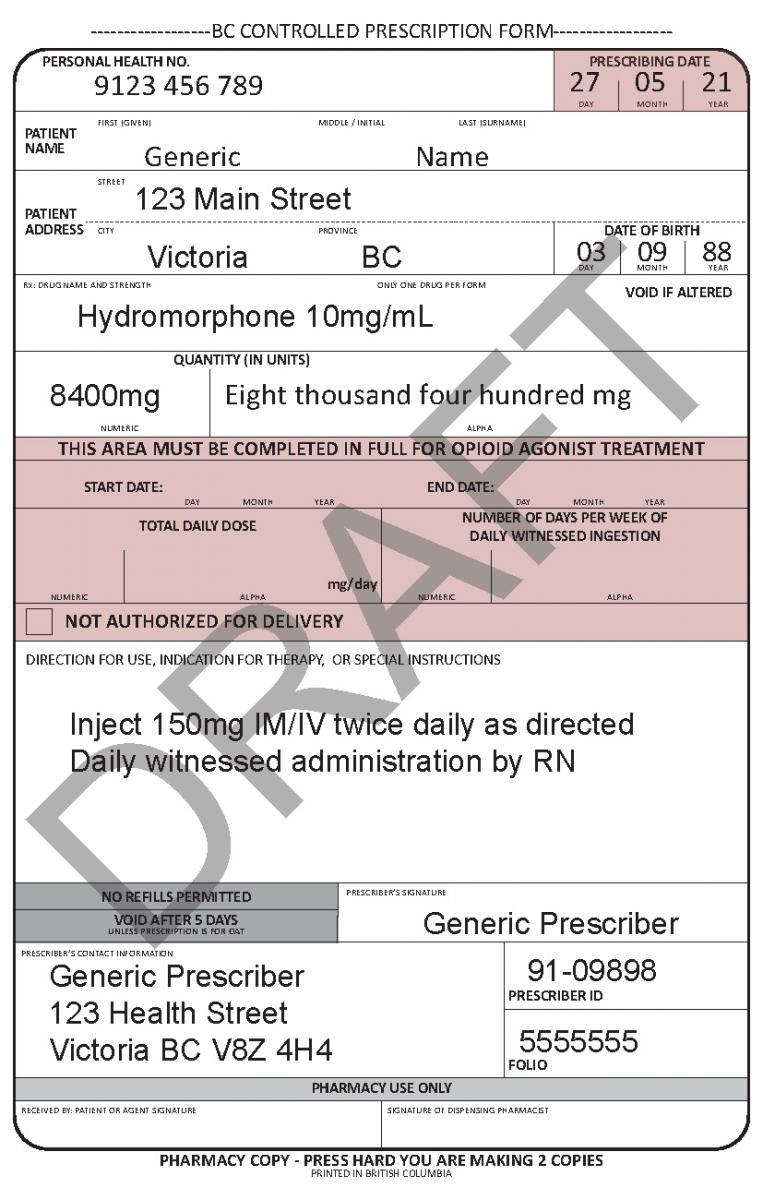
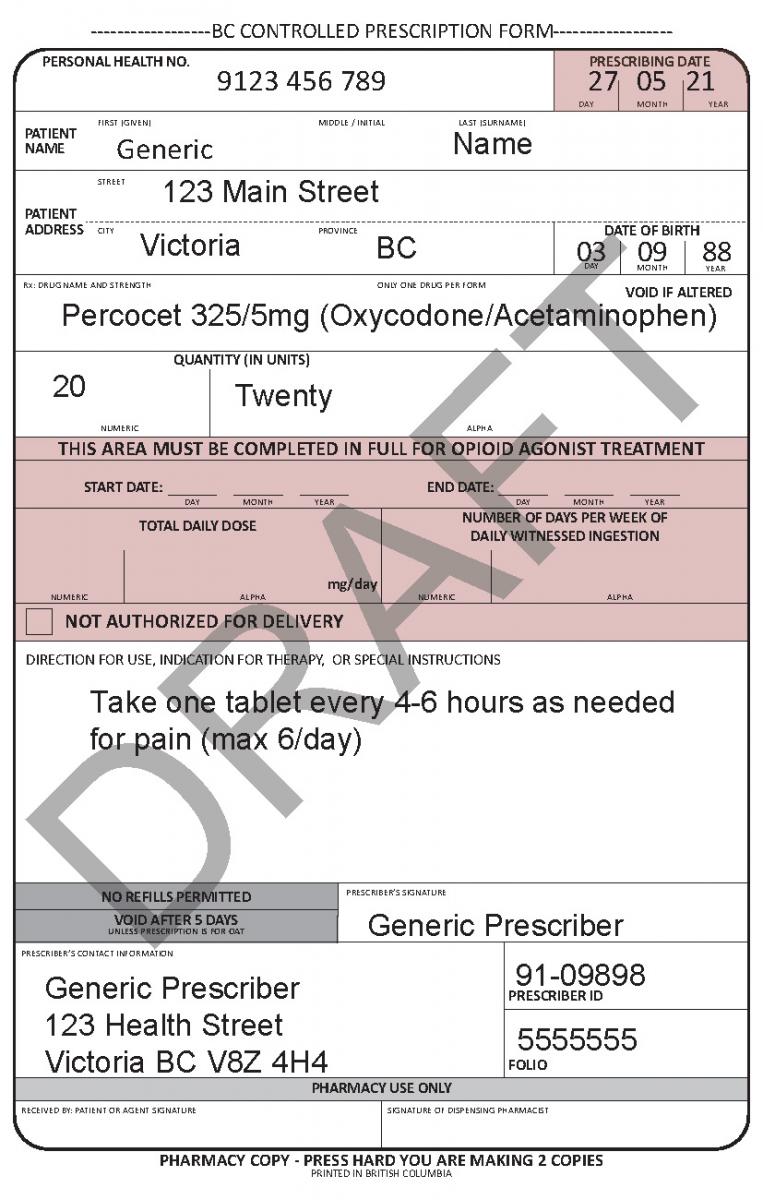
In June 2021, a harmonized Controlled Prescription Program Form was introduced. The harmonized form combines the generic CPP form used for the majority of controlled prescriptions and the methadone CPP form, which is used to prescribe methadone for maintenance treatment.
|
Transition Period The harmonized CPP forms are being gradually phased into practice as prescribers begin to order the harmonized forms. Prescribers can continue to use their existing forms. Pharmacists can accept both the existing CPP forms and the harmonized CPP form. |
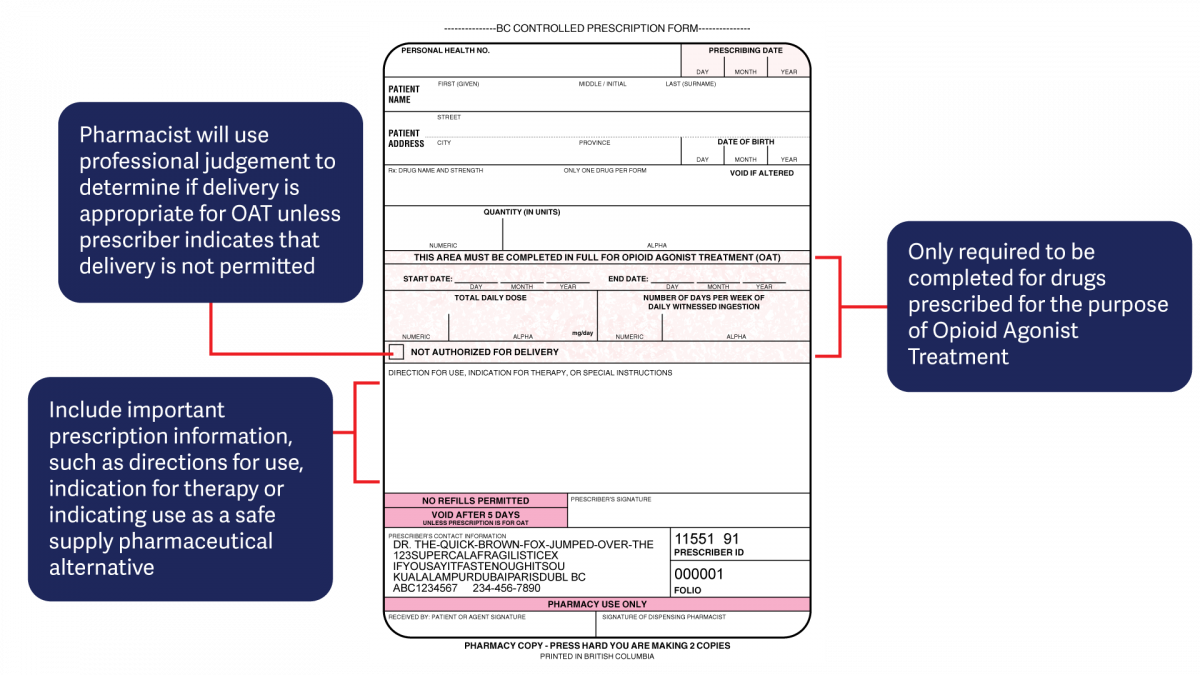
The harmonized CPP form reflects updated Opioid Agonist Treatment (OAT) delivery guidance and no longer requires physician authorization for delivery. Pharmacists are expected to use their professional judgement to determine whether or not to deliver OAT to a patient in accordance with the College of Pharmacists of BC’s Professional Practice Policy – 71 (Delivery of Opioid Agonist Treatment). Prescribers are also able to specify when delivery is not permitted.
See additional information on the development of the new harmonized CPP form
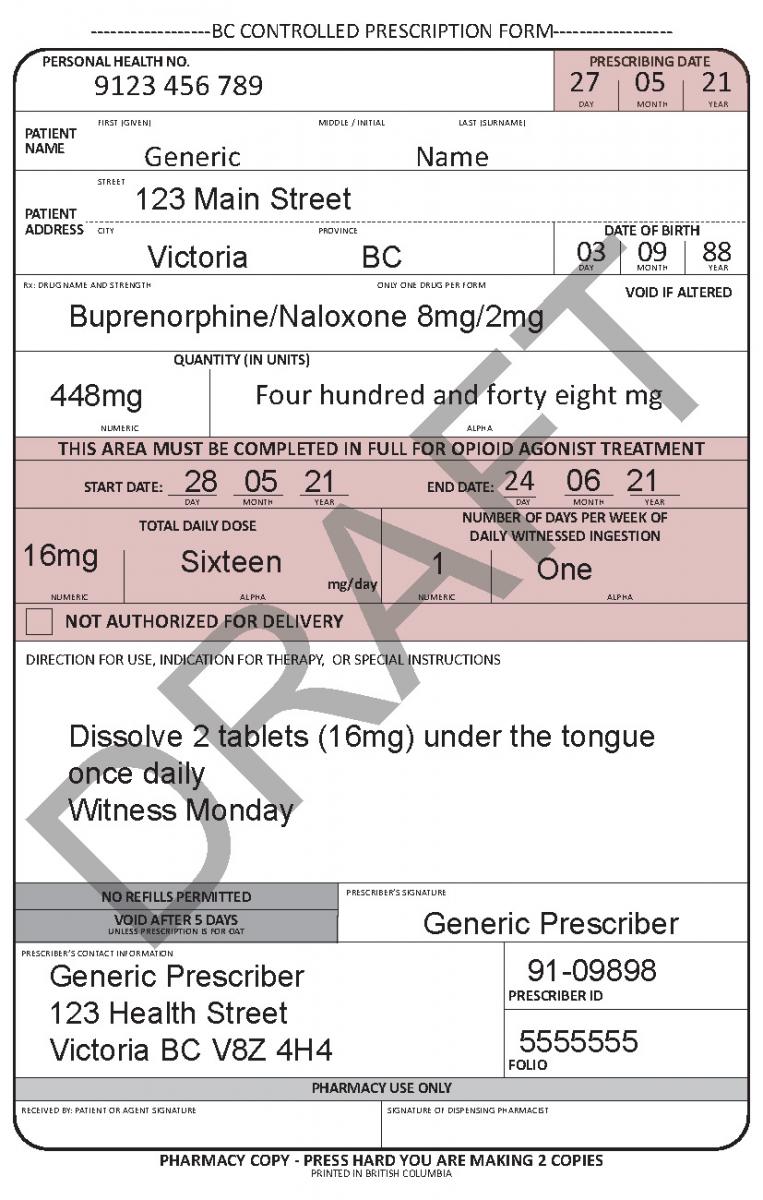
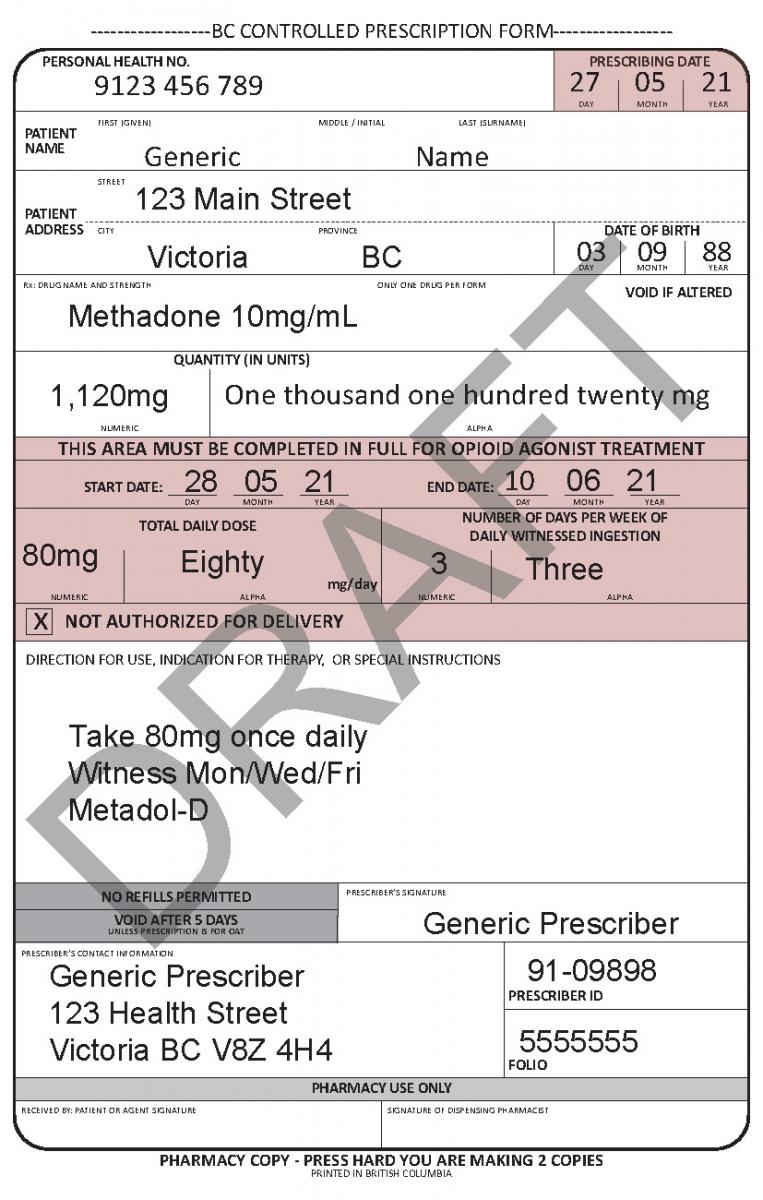
Harmonized Controlled Prescription Program Form
|
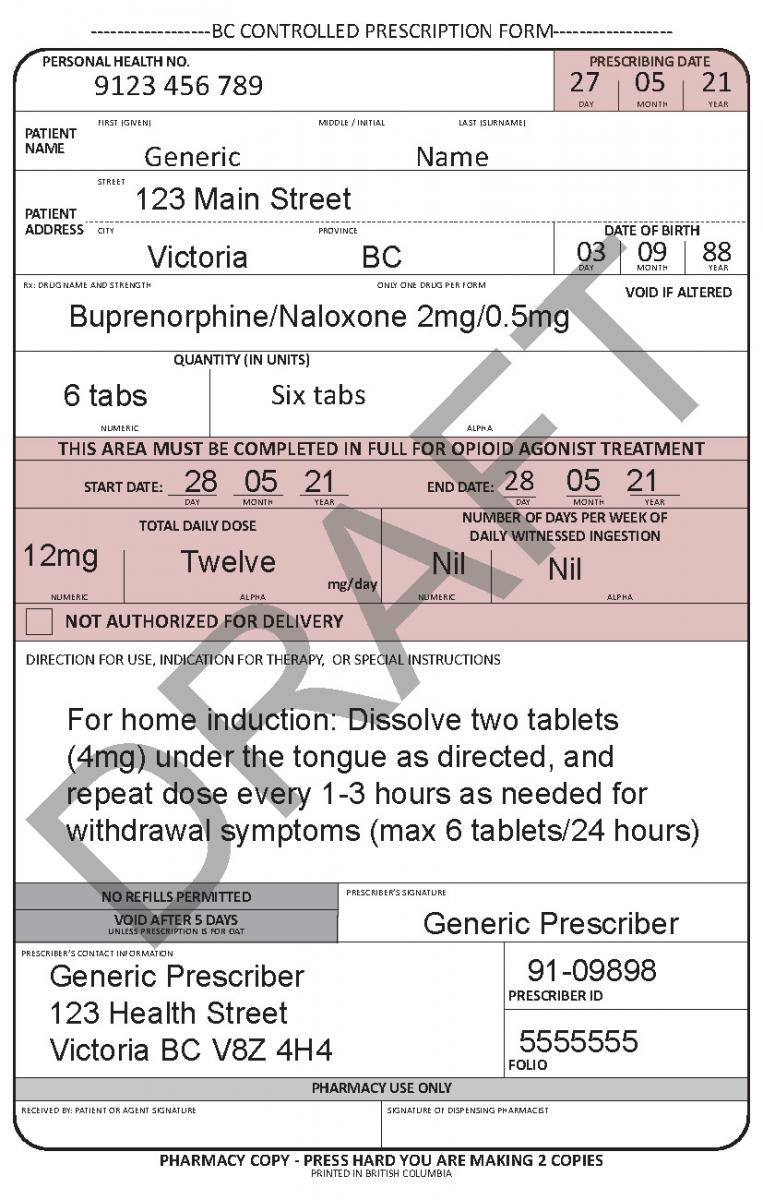
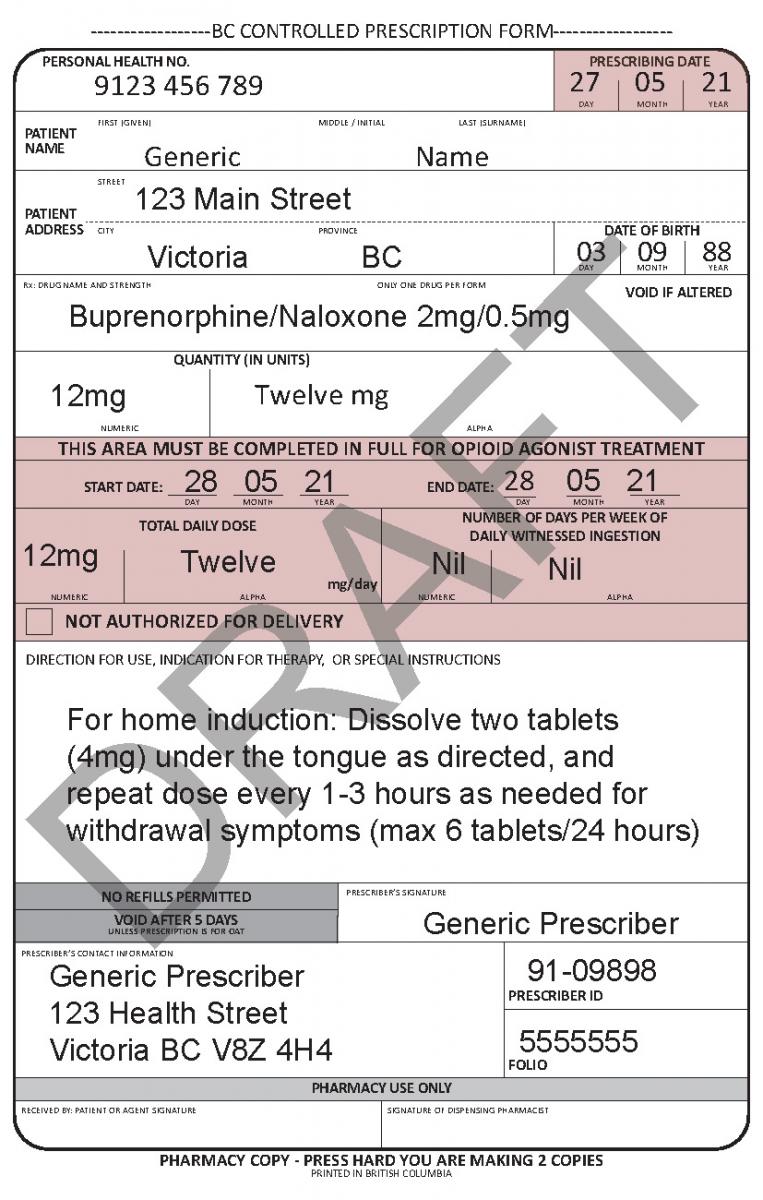
See the following examples of how to use the harmonized CPP form for different types of prescriptions.
For questions on how to complete a CPP form, please reach out to your respective College.
Patient Delivery Options
Unless otherwise indicated, a pharmacist may deliver controlled drugs to a patient based on their professional judgement if they feel delivery is appropriate and in the best interest of the patient.
A prescriber may indicate that delivery is not permitted:
- For drugs prescribed for opioid agonist therapy, prescribers can use the NOT AUTHORIZED FOR DELIVERY option on the new harmonized CPP form.
- For drugs prescribed for opioid agonist therapy using the existing CPP forms, or for other drugs included in the Controlled Prescription Program, prescribers can indicate “Do Not Deliver” on the form or directly communicate with the pharmacist.
Prescribers and Pharmacists should watch for updates on these temporary amendments from their College.
Note: The College of Pharmacists of BC’s Professional Practice Policy-71: Delivery of Opioid Agonist Treatment sets out specific requirements for pharmacists and pharmacy managers working in community pharmacy settings on the delivery of opioid agonist treatment (OAT) drugs by pharmacists directly to patients. This includes a requirement for pharmacists to ensure the decision to deliver or to not deliver (including the rationale for the decision) is reflected in the patient’s record.
Ordering Duplicate Prescription Pads
Duplicate prescription pads are ordered through a prescribers' respective College.
The processing time for orders is 2-3 weeks. To ensure the safety and security of the pads, prescribers should always sign for deliveries of the pads.
Orders should be mailed to the work address listed on file with your College and located within the province of British Columbia. Prescribers need to contact their respective college to change their address for the delivery of the pads.
Note: For prescribers who have multiple work addresses, please contact your College to determine the delivery address. Delivery of the prescription pad to your home address may also be acceptable under extraordinary circumstances, but only after discussion with your College.
Record Retention
Records should be retained in accordance with requirements by respective Colleges.
- For prescribers, do not retain the completed duplicate blue copies in the prescription pad. The duplicate copies should be filed with client/patient health records as per employer’s medical records management policies. Electronic copies of the prescription are acceptable but must be kept with the patient’s record.
- Pharmacists must keep a paper copy - original or faxed - of the CPP prescription form. The paper copy of the prescription must be filed with the patient’s record. Electronic copies of the prescription, if any, must be filed with the dispensing record.
Lost, stolen, or forged prescription pads
Prescribers must ensure secure storage of the prescription pads. If you are concerned that a prescription pad has been lost, stolen, misplaced or forged, or if you did not receive a prescription pad order within specified delivery time, you should contact your respective College as soon as possible.
Prescribers can also report a lost or stolen prescription pad or duplicate prescription pad to PharmaNet Support Services for communication to pharmacies via FanOut.
Voiding a Prescription Pad
Program Contacts
For any specific questions not answered through the Controlled Prescription Program information document, please contact your respective regulatory College for further details.
 Share
Share