
PRP INSIGHTS: Cold Chain Management
During pharmacy reviews, Compliance Officers are confirming that pharmacies have the required equipment to dispense drugs safely and in accordance with manufacturer requirements. This includes cold storage equipment (i.e. refrigerator or freezer) used for cold chain management. Cold chain management refers to the process used to maintain a drug within the required storage temperature range, starting at the manufacturer and ending with the release of the drug to the patient which includes transporting, handling and storage of the drug. Maintaining the cold chain at the pharmacy ensures the integrity, safety and efficacy of the drugs are not compromised, and reduces drug wastage.
Compliance Officers review cold chain management procedures according to Professional Practice Policy – 68: Cold Chain Management (PPP-68). This article discusses some areas where non-compliance has been observed for cold chain management.
-
Minimum/maximum temperatures are not recorded
The current temperature and minimum and maximum temperatures reached since the last temperature recording must be recorded twice daily, preferably at opening and closing. Monitoring and recording of minimum/maximum temperatures is important to ensure that a temperature excursion did not occur between readings. Registrants can achieve this by manual recordings or automatically with a digital temperature monitoring system that records temperatures at a frequency that can determine current temperatures, and minimum and maximum temperatures reached at least twice a day and notifies staff when a temperature excursion occurs. If a temperature excursion occurs, consistent twice daily temperature recordings can also be used to estimate the duration of the excursion. The temperature records of the cold storage equipment must be retained and easily retrievable for at least three years.
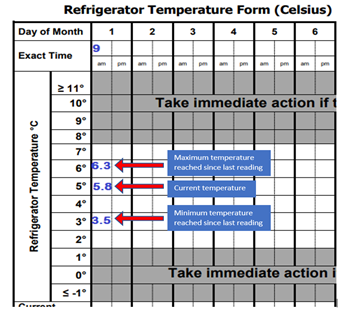
Example of manually recorded minimum/maximum and current temperatures
(Example temperature log adapted from BCCDC's Refrigerator temperature form template)It is expected that pharmacy staff are familiar with the functionality of their digital thermometer or digital temperature monitoring system and know how to retrieve minimum/maximum temperatures as well as reset their devices.
-
A standard “bar” fridge is used
Standard “bar” refrigerators contain a combination refrigerator and freezer component within one compartment. These types of refrigerators are not considered acceptable cold storage equipment as there is a higher likelihood of temperature fluctuations within the compartment. This can lead to unpredictable maintenance of the required cold chain temperatures.
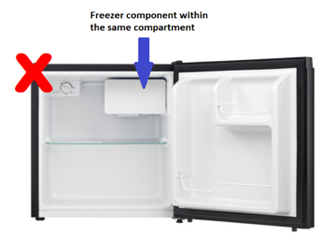
A standard “bar” fridge
-
Written policies and procedures are not in place
Pharmacy managers have a responsibility to ensure appropriate policies and procedures are in place to safely store products once they have been delivered to the pharmacy. As per PPP-68, a pharmacy must establish written policies and procedures that include processes:
- to ensure proper cold chain management,
- to record temperatures of the cold storage equipment in accordance with section 3 of PPP-68,
- to determine and document actions taken when a temperature excursion occurs, and
- for regular maintenance that ensures functionality of cold storage equipment and documenting those processes;
During pharmacy reviews, Compliance Officers will confirm that staff have been trained on these policies and procedures.
To learn more about the Practice Review Program, including how to prepare for your review, visit bcpharmacists.org/prp.
- Practice Review Program, PRP Insights
 Share
Share


